It is hard to realize today what an epoch-making idea it was at that time to dye fabrics with a substance evolved in the laboratory and having no relation whatever to the dyestuffs then known. It was truly the spark of genius which led Perkin to investigate the dyeing properties of that dark-colored precipitate which would have been cast away by any other scientist of that period.... [Schweitzer 482]
 riting the standard chemistry text book of the late nineteenth / early twentieth century, Walter Henry Perkin Jr. (1860-1929) and Stanley Kipping (1863-1949), two distinguished professors of chemistry who happened to be brothers-in-law, explained:
“Although most organic compounds are colourless, some, especially certain classes of the aromatic series, are intensely coloured substances amongst which representatives of almost every shade occur; most of the principal dyes used at the present day are, in fact, aromatic compounds, the primary source of which is coal-tar — hence the well-known expression ‘coal-tar colours.’” (54). In fact, the "well-known expression" they mention here had been current for less than five decades: Perkin's own father had discovered the first of these dyes only in 1856. Inventing a new colour, or, rather, a new way of creating a colour, might not seem very earth-shattering now, but it ranks amongst the most significant scientific discoveries of the Victorian period, as the two professors would have been well aware.
riting the standard chemistry text book of the late nineteenth / early twentieth century, Walter Henry Perkin Jr. (1860-1929) and Stanley Kipping (1863-1949), two distinguished professors of chemistry who happened to be brothers-in-law, explained:
“Although most organic compounds are colourless, some, especially certain classes of the aromatic series, are intensely coloured substances amongst which representatives of almost every shade occur; most of the principal dyes used at the present day are, in fact, aromatic compounds, the primary source of which is coal-tar — hence the well-known expression ‘coal-tar colours.’” (54). In fact, the "well-known expression" they mention here had been current for less than five decades: Perkin's own father had discovered the first of these dyes only in 1856. Inventing a new colour, or, rather, a new way of creating a colour, might not seem very earth-shattering now, but it ranks amongst the most significant scientific discoveries of the Victorian period, as the two professors would have been well aware.
Dyeing was once an expensive and time-consuming business. Natural dyes required specialised processing. In the case of one of the most sought-after, red dye from the cochineal parasite, this started with the preparation of the soil for the cactus plants which harboured them. Then the tiny, delicate cochineals had to be protected from their natural predators, harvested, and powdered in one of several ways. Thousands were needed to make even tiny quantities, with only the female ones yielding "the commercial product, or red tincturial substance on which such value is set" (Simounet 180). Aside from being complicated to make, at the beginning of the nineteenth century such "natural" dyes were only available for a small range of colours. Of this limited range, purple dye was the most expensive of all, its principal source being in the glands of certain marine molluscs. Again, thousands of these were needed to produce small amounts of dye by a costly and, in this case, evil-smelling process. Called Tyrian purple, from the place where it was first made, it was the preserve of the wealthy — of nobility, indeed of royalty.
In such a context, the discovery that the colour could be made from sludgy, oily coal-tar — a plentiful by-product of the coal industry — begins to look more important. As for making an infinite range of colours widely accessible, by straightforward factory methods, that would revolutionise the whole science and industry of colour. More than that, as Hugo Schweitzer explained on the fifty-year Jubilee of the coal-tar industry which this discovery initiated, it had enormous repercussions in almost every branch of life, from agriculture and food processing to cosmetics and, most importantly, to pharmaceuticals and medical science. As Schweitzer concluded, "it is unique in the history of civilization that the honor and credit for the creation of this enormous material and spiritual wealth is unanimously and ungrudgingly accorded to Sir William Henry Perkin" (488).
Perkin's Early Life

Sir William Perkin by Arthur S. Cope, 1906, by kind permission
of the Wellcome Library, London.
William Perkin (1838-1907) was one of the fortunate few who feel drawn to a certain path from a young age. He was born in Shadwell, in the docklands area of the East End of London, at the dawn of the Victorian era. But he was not a child of the slums: his father George was a prosperous builder there, with a gift for music and a deep religious faith, both of which he handed on to his third son William. The boy was sent to private schools, entering the City of London School in 1851. He had many hobbies, from playing the violin and the double bass to model-making, photography and painting. But, as a twelve-year-old schoolboy, he was fascinated when a friend showed him some simple experiments with crystals. He was soon skipping lunch to take extra lessons in chemistry. At fourteen, he started going to listen to Michael Faraday at the Royal Institution. His father encouraged him at first, but saw no prospects in chemical research and tried to interest him in architecture instead. William was not to be dissuaded. At fifteen he enrolled in the recently founded Royal College of Chemistry, to study under its director, the inspiring August Wilhelm von Hoffman (1818-1892). Prince Albert, one of those instrumental in the founding the Royal College, had greatly approved of Hoffman's appointment, and had high hopes of him, particularly in the area of agricultural advance (see Garfield 20-21).
The First Experiments


Left: Entrance to the Royal Society of Chemistry, at Burlington House, Piccadilly. Right: Sir William Perkin's bust, by F. W. Pomeroy, outside the Council Room of the Royal Society (author's photographs).
Scientists at the Royal College already knew that coal tar was potentially useful, and in Scotland Charles Macintosh (1766-1843) had found at least one use for it. Applying a rubbery solution of it to a layer of woollen cloth, and adding another layer of cloth on top of it, he had invented waterproofing. But only a few of the thousands of different chemicals in coal tar had been discovered so far. Hoffman was particularly interested in a liquid called aniline that could be separated out from the benzene in it. He had his own agenda: he hoped it would lead to a way of making quinine to treat malaria — in its natural form, quinine came from the cinchona tree in South America, and was difficult and costly to obtain. Like others at that time, in 1854 William started experimenting on this in a makeshift laboratory at home. Such experiments were risky. Benzene is very flammable, not only burning easily but giving off dangerous fumes. Another of Hoffman's most brilliant students, Charles Mansfield (1819-1855), a close friend and probably the lover of George Meredith's wife Mary Ellen (see Joukovsky), died a long-drawn-out and agonizing death from chemical burns when one of is own experiments went wrong. William, however, was careful — and fortunate. The young researcher proved his father wrong about chemistry by discovering something that would make his and his family's fortune.
A Chance Discovery?
Like many great discoveries, Perkin's was made at least partly by chance. "Chemists have always been desirous of producing natural organic bodies artificially," he explained in a published lecture (4). On this occasion, he himself was trying to make the colourless quinine from a coal-tar product called allyl toluidine. To his surprise, it produced a reddish-brown powder. At this point, curiosity — or "intuitive talent" (Garfield 36) — took over from chance. He tried again, using the simpler aniline this time. Now the residue was black. Persevering, or possibly even trying to clean up (see Dronsfield), he purified and dried the black product, and then treated it with "spirits of wine." Again he was surprised: this resulted in a rich mauve colour. As he told his audience, "this discovery did not in any way originate from a desire to produce a colouring matter, as is sometimes stated, but in experiments of a purely theoretical nature" (4). What sealed his success was what he did next: he tried dyeing a piece of silk with it, and found that it worked brilliantly. Unlike natural dyes, it had been cheap and easy to make, and now it refused to run in water or fade in the sun. It was, in fact, "the first organic dye to be made artificially" (Hart-Davis 240). The young man's friends were impressed; Hoffman was not. This hardly seemed like serious research. Once more, William Perkin followed his own instincts, and once more, he was right to do so.
Fame and Fortune

Coal Tar Works at Greenford in 1858 & 1873, by kind permission
of the Wellcome Library, London.
Still only eighteen, Perkin was allowed to patent his invention, which for a while he called "Tyrian Purple." But, despite its glamorous name, no one was queuing up to try it out: the old dyeing factories were suspicious of it. By now, however, George Perkin had learnt to respect his son's judgement. In 1857, together with William's older brother Thomas, who had fulfilled his father's ambition by studying architecture, he built a dyeworks on Greenford Green, Middlesex, beside the Grand Junction Canal. By 1859, Perkin and Sons were producing and sending out the new dye all over the country, and Perkin had become "one of the first chemists to make his scientific discoveries commercially viable" (Hart-Davis 240).
Perkin was now calling it mauve, from the French word for the similarly-coloured mallow flower — a name not unknown before, but only current from around the end of the eighteenth century, and gaining new currency in this bright new form. The first definition of the noun in the Oxford English Dictionary is: "Any of a range of light shades of purple between lilac and violet; spec. the colour of the aniline dye." As a scientific term for it, Perkin used "mauveine," explaining that it was "composed exclusively of carbon, hydrogen, and nitrogen, in the following proportions: C 27 H 24 N 4" (9), though it was only years later that its structure would be fully understood.

A fashionable gown in an illustration in Godey's Lady's Book (April 1864), np.)
But that was not important in the world of fashion. The colour had got a head start when the fashionable Empress Eugénie, consort of Napoleon III, and then Queen Victoria herself, adopted it, the latter choosing to wear it for her eldest daughter's wedding. In John Philip's painting of The Marriage of Victoria, Princess Royal, 25 January 1858, the monarch is resplendent in a delicate shade of mauve At first the colour came from France, where natural sources had been found for it in lichens (see Garfield 62). But Perkin's analine mauve soon captured the market. Indeed, he himself helped to publicize it by giving lectures on it, and was highly gratified to find Faraday himself in the audience at the Royal Society in 1859 (Garfield 80). The Oxford English Dictionary's first literary citation comes from the same year, and is a quotation from Dickens's All the Year Round. Here, an anonymous contributor praises the dye and its discoverer to the skies. As for the colour itself,
It is rich and pure, and fit for anything; be it fan, slipper, gown, ribbon, handkerchief, tie, or glove. It will lend lustre to the soft changeless twilight of ladies' eyes — it will take any shape to find an excuse to flutter round her cheek — to cling (as the wind blows it) up to her lips — to kiss her foot — to whisper at her ear. O Perkins's [sic] purple, thou art a lucky and a favoured colour! [468]
This is all in the mock-heroic vein, making fun of the world of fashion and particularly this current fad: "We shall soon have purple buses and purple houses" (469). But it does show the extent of the fad. Also cited in the dictionary is the St James's Magazine issue of 1861, the first in a new series of the magazine, which had just been taken on by Mrs S. C. Hall. Two articles deal with it here. In the first, "Mauve and Magenta," the Keeper of Mining Records, Dr. Robert Hunt, discusses its derivation from coal-tar, and is at pains to relate both new colours to the same ultimate sources as natural colouring, that is to say, "to those mysterious forces which — we scarcely yet know how — give colour to Nature. They were produced in the very youth of the world, and have been stored until now in the earth's recesses" (46). The second article, by a Mrs Merrifield, is altogether less scientific and philosophical. Entitled "Use and Abuse of Colours in Dress," it deals with the effect of mauve on the complexion. There is nothing tongue-in-cheek about Merrifield's reference to "the fashionable and really beautiful mauve and its varieties" (292), but she does warn against placing anything of this colour right next to the face, and recommends a light shade for the fairer-skinned (see 292-93).
One special advantage of mauve was its suitability for mourning, which was worn for such long periods in those times. Thus, in 1862, the year following Prince Albert's death, even the bereft Queen Victoria was attracted by it when visiting the International Exhibition at the Crystal Palace in Sydenham. In its write-up of the exhibition, the Times correspondent reported excitedly that a whole block of Perkin's mauve dye was prominently displayed in a glass case in the eastern annexe — "sufficient to dye 300 miles of silk fabric" (2 June 1862, 9). Years later, Perkin's third son Frederick Mollwo Perkin wrote to a former student and historian of the Royal College of Science, sending him a sample of his father's original mauve dye, and also "a small piece of silk which is a portion of a dress accepted by Queen Victoria at the 1862 Exhibition" (qtd. in Garfield 147n.).
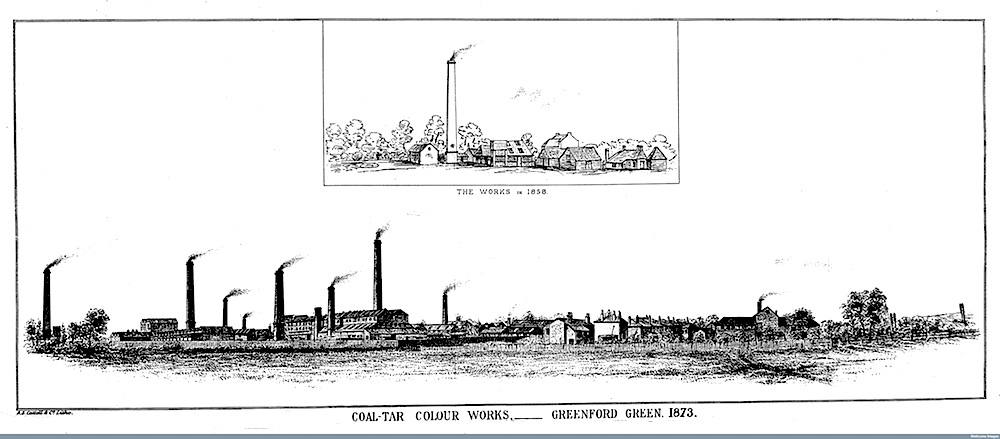
Coal Tar Works at Greenford in 1858 & 1873: Plant belonging to W. H. Perkin and his bother, T. D. Perkin, by kind permission of the Wellcome Library, London.
Mauve, however, was only the beginning. Perkin's patent was not enough to prevent others from using similar methods for different colours, like the magenta mentioned above. Instead of spending all his time and energy trying to battle it out in the courts, Perkin too began to produce new aniline dyes. This was a good move, because, as the anonymous contributor to All the Year Round knew, fads by definition come and go, and even mauve would gradually lose its lustre, if not from the sun, then from the vagaries of fashion. So Perkin diversified and the plant at Greenford grew to dominate the area, and "the Grand Junction Canal turned a different colour every week" (Garfield 81). But elsewhere, too, under the impetus of Perkin's discovery, the aniline dye industry was booming.
Retirement

Sir William Perkin. Source: New York Public Library. Image ID: 1808577.
The way forward was not always straight forward. Apart from patent problems, there were accusations that Perkin's now sprawling manufactory were not simply colouring but polluting the canal, local waterways and the neighbourhood. It is easy to see from the above illustrations how such criticisms were provoked. Worse, after Perkin finally sold the Greenford works at the end of 1873, he quickly became embroiled in an action brought by the new owners, who were unable to run them successfully. Their case was thrown out, but it must have left a bitter taste in Perkin's mouth. Henceforth he carried on his researches quietly at his own laboratory in Sudbury, at the house left vacant when he built a new home in Roxeth, near Harrow-on-the-Hill in Middlesex.
Having been only eighteen at the time of his great discovery, he still had a lifetime of research ahead. He made full use of it, too, and continued to make important contributions in his field, publishing over sixty new papers, "most concerned with magnetic rotary power and and the molecular architecture of increasingly useful chemical compounds (Garfield 124, emphasis added). As was recognised early on, the "thorough and incessant study of this by-product" (coal tar) to which he himself gave such impetus, and in which he himself either led the way or participated, considerably furthered the progress of his discipline in general (see Benedikt 2).
Piously evangelical, charitable, teetotal and vegetarian, Perkin was an unassuming man but was nevertheless disappointed to see how his discovery was exploited abroad as the dye trade at home diminished (see Garfield 120). Still, he continued to be fêted for his introduction of synthetic dyes, which brought hundreds of new colours to the world, brightening up everything from the clothes we wear to the food we eat. Amongst them all, it was the very first that really made his name: when scientists came from far and wide to honour him in America, on the 50-year anniversary of his original discovery, they all wore purple bow-ties. This was also the occasion of the inauguration of the Perkin Medal, first awarded to Perkin himself, then in subsequent years to the most distinguished American chemist of the preceding year.
Besides the medal, he received many other honours and awards in these years, and occupied several important offices: he was president of the Chemical Society of London (1883-85) and of the Society of Chemical Industry (1884–85), and was still president of the Society of Dyers and Colourists when he died. Knighted by King Edward VII in 1906, he passed away in 1907. His first wife, his cousin Jemima, had died of tuberculosis in 1861, but he left behind the two sons from that marriage, and a son (Frederick Mollwo) and three daughters by his Polish second wife Alexandrine, who also outlived him. After all, he was only 69 when he died.
Assessment

Busts of Michael Faraday and William Perkin. These flank the doors of the Council Room of the Royal Society of Chemistry, at the head of the grand staircase.
The news about coal-tar colours was not always good. Workers in the aniline dye industry began to prove susceptible to bladder cancer. There were health scares then, as now: some of the dyes were thought harmful when used in foodstuffs, or irritating in contact with the skin. For good or ill, as Perkin himself was all too well aware, many other scientists were involved in their future development. In particular, there was huge competition from Germany, whose organic chemists had after all laid the groundwork for Perkin's discovery, and who dominated the market from the late 1870s (see McClellan and Dorn 321). This was where "analine bladder cancer" was first noted, and workers in the great dye factories there, as no doubt elsewhere, one day found themselves using their chemicals and chemical know-how to produce explosives and other materials for the war effort.
Still, there was no stopping the advance of this branch of science. Perkin's discovery went on and on yielding good results as well, not only for the textile industry, but in areas such as food colouring, photography, perfumery, and medicine. In the food-colour industry alone, the United States Department of Agriculture's Office of Information pointed out in 1939 that there were now "altogether 49 different certified reds ... 19 different oranges, 16 yellows, 14 blues and 8 greens, as well as a few violets, a brown and a black" (Certified Coal-Tar Colours) — and these were still early days for the industry. The impact on medicine has been particularly wide-ranging. For example, it is impossible to calculate the benefits that have accrued simply from the the use of analine dyes in microscopy. Synthetic colours used in laboratory tests and experiments have also helped in the mass-production of quinine, making malaria treatment more widely available, just as August Hoffman had originally hoped. The diversity of his legacy is astonishing. A hundred years after his birth, Perkin has even been seen as "lighting the path towards rayon, synthetic rubber and other items indelibly linked with a modern age" (Garfield 163).
More generally, there was something else that Perkin could not have known — the far-reaching effect of the revolution he had brought about in the related worlds of organic and manufacturing chemistry. He might not have approved of, let alone taken the credit for, every individual development, but he would doubtless have been proud of one way in which he passed on his gifts. "Perkin the genius begot Perkins of genius," as Dr Benjamin Harrow of Columbia University put it in The Scientific Monthly of 1 September 1919 (245). All three of his sons distinguished themselves in the same field as their father. In particular, in 1912, his eldest son William, who had been a professor at Owens College, Manchester, when he collaborated with Kipping on the textbook, became Waynflete Professor at Oxford, and introduced to that university "the notion and practice of industrial research" (Morrell), sending out his students to become professors of chemistry in their turn to all corners of the country.
"The salts of mauveine are beautifully crystalline," William Perkin said in one of his published lectures (9). He never lost the enthusiasm of the twelve-year-old boy who had gazed in wonder at his friend's experiments, and he passed that enthusiasm on to others, along with the fruits of his own research. No one could believe now, as his father had once done, that this was an unpromising or unprofitable field. Like his own sons, many talented research chemists in future generations would carry on his work, some of them using (and sometimes misusing) his pioneering findings in ways that he could not possibly have foreseen.

Purple-veined flowers of the Common
Mallow (author's photograph).
Related Material
Bibliography
Benedikt, R. The Chemistry of the Coal-Tar Colours. Trans. and ed. E. Knecht. London: George Bell & Sons, 1886. Internet Archive. Uploaded by Google. Web. 28 August 2014.
Certified Coal-Tar Colours. United States Department of Agriculture, Radio Service. 16 October 1939. Uploaded by the National Agricultural Library. Internet Archive. Web. 28 August 2014.
Dronsfield, Alan. "Chemhistory: mauveine." The Reaction (Royal Society of Chemistry site). Web. 12 May 2014.
Garfield, Simon. Mauve: How One Man Invented a Colour That Changed the World. London: Faber, 2000.
Godey's Lady's Book. April 1864. Uploaded by Community Texts. Internet Archive. Web. 28 August 2014.
Harrow, Dr Benjamin. "William Henry Perkin." The Scientific Monthly. Vol. 9 (1 Sept. 1919). Jstor Early Journal Content. Web. 28 August 2014.
Hart-Davis, Adam, ed. Science: The Definitive Visual Guide. London, New York etc.: Dorling Kindersley, 2012.
Joukovsky, Nicholas. "Mary Ellen's First Affair: New Light on the Biographical Background to 'Modern Love,'" The Times Literary Supplement, 15 June 2007, 13-15.
McClellan, James E. III, and Harold Dorn. Science and Technology in World History: An Introduction. Baltimore: Johns Hopkins University Press, 2008.
Merrifield, Mrs. "The Use and Abuse of Colours in Dress." St James's Magazine. Vol. I (April-July 1861): 289-295. Uploaded from European Libraries. Internet Archive. Web. 28 August 2014.
Morrell, Jack. "William Henry Perkin (1860-1929)." Oxford Dictionary of National Biography. Online ed. 28 August 2014.
Oxford English Dictionary. See entries on "mauve."
Perkin, W. H. On the Aniline or Coal Tar Colours (Cantor Lectures). London: Trounce (printers), 1869. Uploaded from the California Digital Library. Internet Archive. Web. 28 August 2014.
Perkin, W. H., Jun. and F. Stanley Kipping. Organic Chemistry:. New and revised ed. London and Edinburgh: W. and R. Chambers, 1908. Uploaded by the California Digital Library. Internet Archive. Web. 28 August 2014.
"Perkins' Purple." All the Year Round. 10 Sept. 1869: 468-71. Google Books. Web. 28 August 2014.
Schweitzer, Hugo. "The Influence of Sir William Henry Perkin's Discovery upon Our Science." Science. Vol. XXIV. No. 616 (19 Oct. 1906): 481-88. Jstor Early Journal Content. Web. 28 August 2014.
Simounet, M. "Importation of Cochineal into Algiers: Description, Rearing, and Propogation of This Insect." The Chemist, Or, Reporter of Chemical Discoveries and Improvements, and Protector of the Rights of the Chemist and Chemical Manufacturer. Eds. Charles Watt and John Watt. Vol. V (Vol.II, New Series). London: Alexander Watt, 1844. 179-81.
"Sir William Perkin and the American Jubilee of the Coal Tar Industry." Science. Vol. XXIV. No. 613 (28 Sept. 1906): 413-14. Jstor Early Journal Content. Web. 28 August 2014.
Times Digital Archive. Web. 28 August 2014.
Travis, Anthony S. "Perkin, Sir William Henry (1838-1907)." Oxford Dictionary of National Biography. Online ed. 28 August 2014.
Last modified 28 August 2014